Introductory Astronomy:
Ozone Depletion
A Good Ozone Link
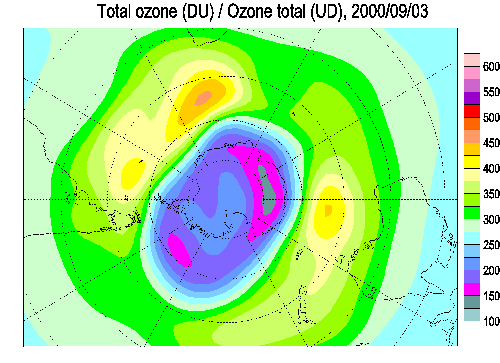
Sunlight creates new ozone from O2 by breaking the bond
with a UV photon. The liberated O atoms are then free to join with
another O2 atom to for ozone (O3). Arctic
regions, however, go without sunlight for months at a time. During
these periods, ozone is destroyed by a cyclic reaction with Chlorine
(Cl) atoms that come from chemicals called cholorofluorocarbons, or
CFCs, that were used in air conditioners and refrigerators in the
U.S., until recently. Each Cl atom can destroy tens to hundreds of
thousands of ozone molecules before it is reincorporated into a more
inert form.
CFCl3
+ UV Light ==> CFCl2 + Cl
Cl + O3 ==> ClO + O2
ClO + O ==> Cl + O2
The
free chlorine atom is then free to attack another ozone molecule
Cl
+ O3 ==> ClO + O2
ClO + O ==> Cl + O2
and
again ...
Cl
+ O3 ==> ClO + O2
ClO + O ==> Cl + O2
and
again... for thousands of times.


